| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:35 UTC |
|---|
| HMDB ID | HMDB0001301 |
|---|
| Secondary Accession Numbers | - HMDB0002240
- HMDB01301
- HMDB02240
|
|---|
| Metabolite Identification |
|---|
| Common Name | 1-Pyrroline-5-carboxylic acid |
|---|
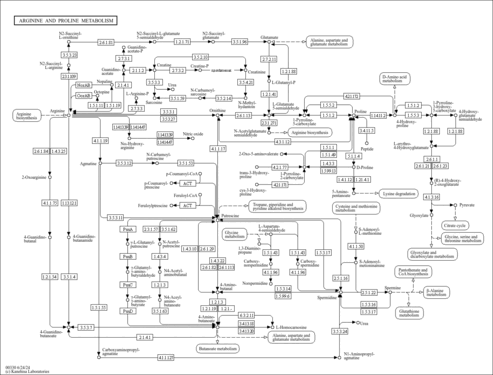
| Description | 1-Pyrroline-5-carboxylic acid (CAS: 2906-39-0) is an enamine or an imino acid that forms upon the spontaneous dehydration of L-glutamate gamma-semialdehyde in aqueous solutions. The stereoisomer (S)-1-pyrroline-5-carboxylate is an intermediate in glutamate metabolism, arginine degradation, and proline biosynthesis and degradation. It can also be converted into or be formed from three amino acids: L-glutamate, L-ornithine, and L-proline. In particular, it is synthesized via the oxidation of proline by pyrroline-5-carboxylate reductase 1 (PYCR1) (EC 1.5.1.2) or by proline dehydrogenase (PRODH) (EC 1.5.99.8). It is hydrolyzed into L-glutamate by delta-1-pyrroline-5-carboxylate dehydrogenase (ALDH4A1) (EC 1.5.1.12). It is also one of the few metabolites that can act as a precursor to other metabolites of both the urea cycle and the tricarboxylic acid (TCA) cycle. Under certain conditions, pyrroline-5-carboxylate can act as a neurotoxin and a metabotoxin. A neurotoxin causes damage to nerve cells and nerve tissues. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of pyrroline-5-carboxylate are associated with at least five inborn errors of metabolism, including hyperprolinemia type I, hyperprolinemia type II, iminoglycinuria, prolinemia type II, and pyruvate carboxylase deficiency. Hyperprolinemia type II results in high levels of pyrroline-5-carboxylate. People with hyperprolinemia type II have signs and symptoms that vary in severity, but they are more likely than type I to have seizures or intellectual disability. Pyrroline-5-carboxylate is highly reactive and excess quantities have been shown to cause cell death and apoptosis (PMID: 15548746 ). |
|---|
| Structure | InChI=1S/C5H7NO2/c7-5(8)4-2-1-3-6-4/h3-4H,1-2H2,(H,7,8)/t4-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Pyrroline-5-carboxylate | ChEBI, KEGG | | L-1-Pyrroline-5-carboxylate | ChEBI, KEGG | | 1-Pyrroline-5-carboxylic acid | Generator | | (S)-1-Pyrroline-5-carboxylic acid | Generator | | L-1-Pyrroline-5-carboxylic acid | Generator | | delta-1-Pyrroline-5-carboxylate | MeSH | | delta-1-Pyrroline-5-carboxylate, (+-)-isomer | MeSH | | delta-1-Pyrroline-5-carboxylic acid | MeSH | | delta-1-Pyrroline-5-carboxylate, 14C-labeled, (+-)-isomer | MeSH | | delta(1)Pyrroline-5-carboxylate | MeSH | | Pyrroline-5-carboxylate | MeSH | | (2S)-3,4-Dihydro-2H-pyrrole-2-carboxylic acid | HMDB | | (S)-1-Pyrroline-5-carboxylate | HMDB | | 3,4-Dihydro-2H-pyrrole-2-carboxylic acid | HMDB | | delta1-Pyrroline-5-carboxylic acid | HMDB | | Δ1-Pyrroline-5-carboxylic acid | HMDB |
|
|---|
| Chemical Formula | C5H7NO2 |
|---|
| Average Molecular Weight | 113.1146 |
|---|
| Monoisotopic Molecular Weight | 113.047678473 |
|---|
| IUPAC Name | (2S)-3,4-dihydro-2H-pyrrole-2-carboxylic acid |
|---|
| Traditional Name | L-1-pyrroline-5-carboxylate |
|---|
| CAS Registry Number | 64199-88-8 |
|---|
| SMILES | OC(=O)[C@@H]1CCC=N1 |
|---|
| InChI Identifier | InChI=1S/C5H7NO2/c7-5(8)4-2-1-3-6-4/h3-4H,1-2H2,(H,7,8)/t4-/m0/s1 |
|---|
| InChI Key | DWAKNKKXGALPNW-BYPYZUCNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon), or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid or derivatives
- Pyrroline carboxylic acid
- Pyrroline carboxylic acid or derivatives
- Pyrroline
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organoheterocyclic compound
- Azacycle
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Carbonyl group
- Imine
- Organic nitrogen compound
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 1.79 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 8.6329 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 4.43 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 292.7 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 707.8 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 314.3 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 78.8 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 207.5 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 72.9 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 253.7 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 270.1 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 378.8 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 595.9 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 120.1 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 737.3 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 194.1 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 212.4 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 649.6 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 305.3 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 265.9 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized |
|---|
| General References | - Simila S: Hydroxyproline metabolism in type II hyperprolinaemia. Ann Clin Biochem. 1979 Jul;16(4):177-81. [PubMed:533224 ]
- Humbertclaude V, Rivier F, Roubertie A, Echenne B, Bellet H, Vallat C, Morin D: Is hyperprolinemia type I actually a benign trait? Report of a case with severe neurologic involvement and vigabatrin intolerance. J Child Neurol. 2001 Aug;16(8):622-3. [PubMed:11510941 ]
- Mixson AJ, Phang JM: The uptake of pyrroline 5-carboxylate. Group translocation mediating the transfer of reducing-oxidizing potential. J Biol Chem. 1988 Aug 5;263(22):10720-4. [PubMed:3392037 ]
- Wakabayashi Y: Tissue-selective expression of enzymes of arginine synthesis. Curr Opin Clin Nutr Metab Care. 1998 Jul;1(4):335-9. [PubMed:10565370 ]
- Onenli-Mungan N, Yuksel B, Elkay M, Topaloglu AK, Baykal T, Ozer G: Type II hyperprolinemia: a case report. Turk J Pediatr. 2004 Apr-Jun;46(2):167-9. [PubMed:15214748 ]
- Fleming GA, Hagedorn CH, Granger AS, Phang JM: Pyrroline-5-carboxylate in human plasma. Metabolism. 1984 Aug;33(8):739-42. [PubMed:6748947 ]
- Deuschle K, Funck D, Forlani G, Stransky H, Biehl A, Leister D, van der Graaff E, Kunze R, Frommer WB: The role of [Delta]1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell. 2004 Dec;16(12):3413-25. doi: 10.1105/tpc.104.023622. Epub 2004 Nov 17. [PubMed:15548746 ]
|
|---|