| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2009-02-02 11:59:47 UTC |
|---|
| Update Date | 2022-03-07 02:51:12 UTC |
|---|
| HMDB ID | HMDB0011644 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol |
|---|
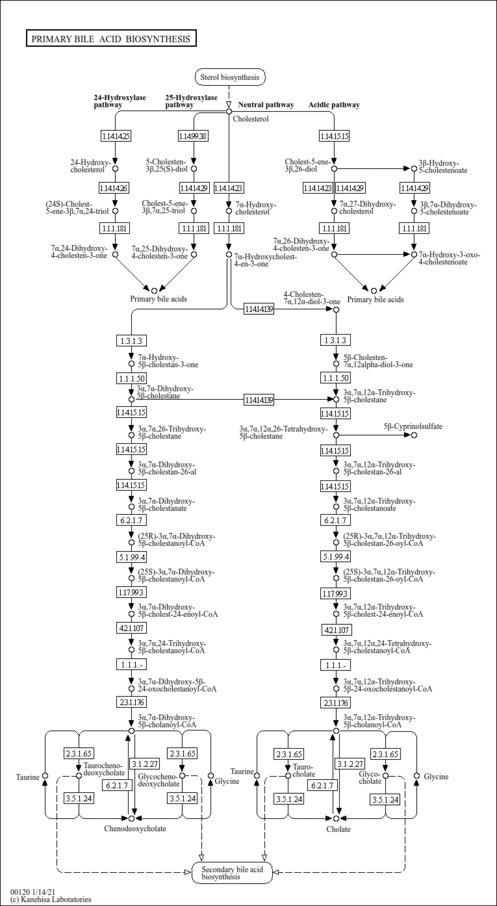
| Description | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol is a hydroxysterol and a bile acid intermediate. It is produced from the reaction of 24(R)-Hydroxycholesterol with the enzyme CYP39A, which is also known as 24-hydroxycholesterol 7alpha-hydroxylase (EC 1.14.13.99). This enzyme catalyzes the following reaction: (24R)-cholest-5-ene-3beta,24-diol + NADPH + H+ O2 = (24R)-cholest-5-ene-3beta,7alpha,24-triol + NADP+ + H2O. This leads to the 7-alpha hydroxylation of 24(R)-hydroxycholesterol. This enzyme can act on both the 24R and 24S isomers. |
|---|
| Structure | [H][C@@]12CCC([C@H](C)CC[C@@H](O)C(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](O)C=C2C[C@@H](O)CC[C@]12C InChI=1S/C27H46O3/c1-16(2)23(29)9-6-17(3)20-7-8-21-25-22(11-13-27(20,21)5)26(4)12-10-19(28)14-18(26)15-24(25)30/h15-17,19-25,28-30H,6-14H2,1-5H3/t17-,19+,20?,21+,22+,23-,24-,25+,26+,27-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (24R)-Cholest-5-ene-3-b,7-a,24-triol | Generator | | (24R)-Cholest-5-ene-3-β,7-α,24-triol | Generator | | (24R)-Cholest-5-ene 3-b,7-a,24-triol | HMDB | | (24R)-Cholest-5-ene-3beta,7alpha,24-triol | HMDB | | 5a-Cholesta-7,24-dien-3-b-ol | HMDB | | 5a-Cholesta-7,24-dien-3-beta-ol | HMDB |
|
|---|
| Chemical Formula | C27H46O3 |
|---|
| Average Molecular Weight | 418.6523 |
|---|
| Monoisotopic Molecular Weight | 418.344695338 |
|---|
| IUPAC Name | (1S,2R,5S,9S,10S,11S,15R)-14-[(2R,5R)-5-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-ene-5,9-diol |
|---|
| Traditional Name | (1S,2R,5S,9S,10S,11S,15R)-14-[(2R,5R)-5-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-ene-5,9-diol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]12CCC([C@H](C)CC[C@@H](O)C(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](O)C=C2C[C@@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C27H46O3/c1-16(2)23(29)9-6-17(3)20-7-8-21-25-22(11-13-27(20,21)5)26(4)12-10-19(28)14-18(26)15-24(25)30/h15-17,19-25,28-30H,6-14H2,1-5H3/t17-,19+,20?,21+,22+,23-,24-,25+,26+,27-/m1/s1 |
|---|
| InChI Key | ZNCHPOYZMVVJCK-DPFMVWRKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as trihydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears three hydroxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Trihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Trihydroxy bile acid, alcohol, or derivatives
- 24-hydroxysteroid
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- 7-hydroxysteroid
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Hydroxysteroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. | 7.65 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 17.0654 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 2.35 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 59.6 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 3211.9 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 267.1 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 233.5 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 172.5 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 527.1 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 818.7 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 733.7 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 97.1 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 1362.0 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 604.9 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 1777.6 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 449.2 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 497.9 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 197.1 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 383.8 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 8.3 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,1TMS,isomer #1 | CC(C)[C@@H](CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O)C=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)O[Si](C)(C)C | 3474.2 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,1TMS,isomer #2 | CC(C)[C@H](O)CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O[Si](C)(C)C)C=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C | 3474.8 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,1TMS,isomer #3 | CC(C)[C@H](O)CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O)C=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@]12C | 3478.5 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,2TMS,isomer #1 | CC(C)[C@@H](CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O[Si](C)(C)C)C=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)O[Si](C)(C)C | 3455.3 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,2TMS,isomer #2 | CC(C)[C@@H](CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O)C=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@]12C)O[Si](C)(C)C | 3495.5 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,2TMS,isomer #3 | CC(C)[C@H](O)CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O[Si](C)(C)C)C=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@]12C | 3406.8 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,3TMS,isomer #1 | CC(C)[C@@H](CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O[Si](C)(C)C)C=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@]12C)O[Si](C)(C)C | 3407.6 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,1TBDMS,isomer #1 | CC(C)[C@@H](CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O)C=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)O[Si](C)(C)C(C)(C)C | 3729.5 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,1TBDMS,isomer #2 | CC(C)[C@H](O)CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O[Si](C)(C)C(C)(C)C)C=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C | 3708.9 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,1TBDMS,isomer #3 | CC(C)[C@H](O)CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O)C=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@]12C | 3706.9 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,2TBDMS,isomer #1 | CC(C)[C@@H](CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O[Si](C)(C)C(C)(C)C)C=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)O[Si](C)(C)C(C)(C)C | 3929.7 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,2TBDMS,isomer #2 | CC(C)[C@@H](CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O)C=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@]12C)O[Si](C)(C)C(C)(C)C | 3959.9 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,2TBDMS,isomer #3 | CC(C)[C@H](O)CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O[Si](C)(C)C(C)(C)C)C=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@]12C | 3850.4 | Semi standard non polar | 33892256 | | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol,3TBDMS,isomer #1 | CC(C)[C@@H](CC[C@@H](C)C1CC[C@H]2[C@@H]3[C@H](O[Si](C)(C)C(C)(C)C)C=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@]12C)O[Si](C)(C)C(C)(C)C | 4075.9 | Semi standard non polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-1239400000-cd1b8c75eaf66591d8c2 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol GC-MS (3 TMS) - 70eV, Positive | splash10-00xu-2110149000-138cf2097993192ea364 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 10V, Positive-QTOF | splash10-0ue9-0007900000-17814845dbf0bec54cfd | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 20V, Positive-QTOF | splash10-0f89-3009400000-fd95622ed3f4f1fae1c4 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 40V, Positive-QTOF | splash10-00fr-5029000000-70e1fb26560a91668d82 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 10V, Negative-QTOF | splash10-014i-0005900000-a892257c0a1e6d75d1d9 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 20V, Negative-QTOF | splash10-00kb-0009500000-66d60259ffcab13684b4 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 40V, Negative-QTOF | splash10-0fl9-7009200000-99af00a0dfd9097d149b | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 10V, Negative-QTOF | splash10-014i-0000900000-f2cb6399034352e924c8 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 20V, Negative-QTOF | splash10-014i-1002900000-b6143eba8eae9ed9668b | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 40V, Negative-QTOF | splash10-014j-3019800000-4bfcf5c6acaa256d7599 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 10V, Positive-QTOF | splash10-0zgi-0308900000-66129b219db1313f7273 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 20V, Positive-QTOF | splash10-001i-3329100000-91ec216cab7fcf837baf | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol 40V, Positive-QTOF | splash10-0adi-3943000000-12a20d116574d822ddef | 2021-09-22 | Wishart Lab | View Spectrum |
|
|---|