| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:00 UTC |
|---|
| HMDB ID | HMDB0000235 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Thiamine |
|---|
| Description | Thiamine, also known as aneurin or vitamin B1, belongs to the class of organic compounds known as thiamines. Thiamines are compounds containing a thiamine moiety, which is structurally characterized by a 3-[(4-Amino-2-methyl-pyrimidin-5-yl)methyl]-4-methyl-thiazol-5-yl backbone. Thiamine exists in all living species, ranging from bacteria to plants to humans. Thiamine biosynthesis occurs in bacteria, some protozoans, plants, and fungi. Thiamine is a vitamin and an essential nutrient meaning the body cannot synthesize it, and it must be obtained from the diet. It is soluble in water and insoluble in alcohol. Thiamine decomposes if heated. Thiamine was first discovered in 1897 by Umetaro Suzuki in Japan when researching how rice bran cured patients of Beriberi. Thiamine was the first B vitamin to be isolated in 1926 and was first made in 1936. Thiamine plays a key role in intracellular glucose metabolism and it is thought that thiamine inhibits the effect of glucose and insulin on arterial smooth muscle cell proliferation. Thiamine plays an important role in helping the body convert carbohydrates and fat into energy. It is essential for normal growth and development and helps to maintain proper functioning of the heart and the nervous and digestive systems. Thiamine cannot be stored in the body; however, once absorbed, the vitamin is concentrated in muscle tissue. Thiamine has antioxidant, erythropoietic, cognition-and mood-modulatory, antiatherosclerotic, putative ergogenic, and detoxification activities. Natural derivatives of thiamine, such as thiamine monophosphate (ThMP), thiamine diphosphate (ThDP), also sometimes called thiamine pyrophosphate (TPP), thiamine triphosphate (ThTP), and adenosine thiamine triphosphate (AThTP), act as coenzymes in addition to performing unique biological functions. Thiamine deficiency can lead to beriberi, Wernicke-Korsakoff syndrome, optic neuropathy, Leigh's disease, African seasonal ataxia (or Nigerian seasonal ataxia), and central pontine myelinolysis. In Western countries, thiamine deficiency is seen mainly in chronic alcoholism. Thiamine supplements or thiamine therapy can be used for the treatment of a number of disorders including thiamine and niacin deficiency states, Korsakov's alcoholic psychosis, Wernicke-Korsakov syndrome, delirium, and peripheral neuritis. In humans, thiamine is involved in the metabolic disorder called 2-methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency. Outside of the human body, Thiamine is found in high quantities in whole grains, legumes, pork, fruits, and yeast and fish. Grain processing removes much of the thiamine content in grains, so in many countries cereals and flours are enriched with thiamine. |
|---|
| Structure | CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N InChI=1S/C12H17N4OS/c1-8-11(3-4-17)18-7-16(8)6-10-5-14-9(2)15-12(10)13/h5,7,17H,3-4,6H2,1-2H3,(H2,13,14,15)/q+1 |
|---|
| Synonyms | | Value | Source |
|---|
| 3-(4-AMINO-2-methyl-pyrimidin-5-ylmethyl)-5-(2-hydroxy-ethyl)-4-methyl-thiazol-3-ium | ChEBI | | Aneurin | ChEBI | | Antiberiberi factor | ChEBI | | Thiamin | ChEBI | | Thiamine(1+) ion | ChEBI | | Thiaminium | ChEBI | | Vitamin b1 | ChEBI | | Apate drops | HMDB | | Beatine | HMDB | | Bedome | HMDB | | Begiolan | HMDB | | Benerva | HMDB | | Bequin | HMDB | | Berin | HMDB | | Betalin S | HMDB | | Betaxin | HMDB | | Bethiazine | HMDB | | Beuion | HMDB | | Bevitex | HMDB | | Bevitine | HMDB | | Bewon | HMDB | | Biamine | HMDB | | Bithiamin | HMDB | | Biuno | HMDB | | Bivatin | HMDB | | Bivita | HMDB | | Cernevit-12 | HMDB | | Clotiamina | HMDB | | Eskapen | HMDB | | Eskaphen | HMDB | | Hybee | HMDB | | Lixa-beta | HMDB | | Metabolin | HMDB | | Slowten | HMDB | | THD | HMDB | | Thiadoxine | HMDB | | Thiaminal | HMDB | | Thiamol | HMDB | | Thiavit | HMDB | | Tiamidon | HMDB | | Tiaminal | HMDB | | Trophite | HMDB | | Vetalin S | HMDB | | VIB | HMDB | | Vinothiam | HMDB | | Vitaneuron | HMDB | | Mononitrate, thiamine | MeSH, HMDB | | Thiamine mononitrate | MeSH, HMDB | | Vitamin b 1 | MeSH, HMDB |
|
|---|
| Chemical Formula | C12H17N4OS |
|---|
| Average Molecular Weight | 265.355 |
|---|
| Monoisotopic Molecular Weight | 265.112306876 |
|---|
| IUPAC Name | 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium |
|---|
| Traditional Name | thiamine |
|---|
| CAS Registry Number | 70-16-6 |
|---|
| SMILES | CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N |
|---|
| InChI Identifier | InChI=1S/C12H17N4OS/c1-8-11(3-4-17)18-7-16(8)6-10-5-14-9(2)15-12(10)13/h5,7,17H,3-4,6H2,1-2H3,(H2,13,14,15)/q+1 |
|---|
| InChI Key | JZRWCGZRTZMZEH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as thiamines. Thiamines are compounds containing a thiamine moiety, which is structurally characterized by a 3-[(4-Amino-2-methyl-pyrimidin-5-yl)methyl]-4-methyl-thiazol-5-yl backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Thiamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiamine
- 4,5-disubstituted 1,3-thiazole
- Aminopyrimidine
- Imidolactam
- Azole
- Thiazole
- Heteroaromatic compound
- Azacycle
- Alcohol
- Organopnictogen compound
- Primary amine
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Amine
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 248 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 500 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections| Adduct Type | Data Source | CCS Value (Å2) | Reference |
|---|
| [M-H]- | MetCCS_train_neg | 160.3 | 30932474 | | [M+H]+ | MetCCS_test_pos | 162.373 | 30932474 |
|
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.19 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.041 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 5.68 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 338.6 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 440.8 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 210.9 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 83.2 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 150.3 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 44.6 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 289.3 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 262.5 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 807.2 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 581.9 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 40.6 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 828.1 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 169.3 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 179.3 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 409.8 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 353.2 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 297.3 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Thiamine,1TMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C)=C2C)C(N)=N1 | 2471.7 | Semi standard non polar | 33892256 | | Thiamine,1TMS,isomer #2 | CC1=NC=C(C[N+]2=CSC(CCO)=C2C)C(N[Si](C)(C)C)=N1 | 2549.4 | Semi standard non polar | 33892256 | | Thiamine,2TMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C)=C2C)C(N[Si](C)(C)C)=N1 | 2502.4 | Semi standard non polar | 33892256 | | Thiamine,2TMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C)=C2C)C(N[Si](C)(C)C)=N1 | 2467.2 | Standard non polar | 33892256 | | Thiamine,2TMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C)=C2C)C(N[Si](C)(C)C)=N1 | 3264.5 | Standard polar | 33892256 | | Thiamine,2TMS,isomer #2 | CC1=NC=C(C[N+]2=CSC(CCO)=C2C)C(N([Si](C)(C)C)[Si](C)(C)C)=N1 | 2540.1 | Semi standard non polar | 33892256 | | Thiamine,2TMS,isomer #2 | CC1=NC=C(C[N+]2=CSC(CCO)=C2C)C(N([Si](C)(C)C)[Si](C)(C)C)=N1 | 2549.2 | Standard non polar | 33892256 | | Thiamine,2TMS,isomer #2 | CC1=NC=C(C[N+]2=CSC(CCO)=C2C)C(N([Si](C)(C)C)[Si](C)(C)C)=N1 | 3162.5 | Standard polar | 33892256 | | Thiamine,3TMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C)=C2C)C(N([Si](C)(C)C)[Si](C)(C)C)=N1 | 2514.5 | Semi standard non polar | 33892256 | | Thiamine,3TMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C)=C2C)C(N([Si](C)(C)C)[Si](C)(C)C)=N1 | 2583.7 | Standard non polar | 33892256 | | Thiamine,3TMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C)=C2C)C(N([Si](C)(C)C)[Si](C)(C)C)=N1 | 2946.7 | Standard polar | 33892256 | | Thiamine,1TBDMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C(C)(C)C)=C2C)C(N)=N1 | 2740.7 | Semi standard non polar | 33892256 | | Thiamine,1TBDMS,isomer #2 | CC1=NC=C(C[N+]2=CSC(CCO)=C2C)C(N[Si](C)(C)C(C)(C)C)=N1 | 2747.4 | Semi standard non polar | 33892256 | | Thiamine,2TBDMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C(C)(C)C)=C2C)C(N[Si](C)(C)C(C)(C)C)=N1 | 2905.4 | Semi standard non polar | 33892256 | | Thiamine,2TBDMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C(C)(C)C)=C2C)C(N[Si](C)(C)C(C)(C)C)=N1 | 2897.6 | Standard non polar | 33892256 | | Thiamine,2TBDMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C(C)(C)C)=C2C)C(N[Si](C)(C)C(C)(C)C)=N1 | 3365.0 | Standard polar | 33892256 | | Thiamine,2TBDMS,isomer #2 | CC1=NC=C(C[N+]2=CSC(CCO)=C2C)C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=N1 | 2927.6 | Semi standard non polar | 33892256 | | Thiamine,2TBDMS,isomer #2 | CC1=NC=C(C[N+]2=CSC(CCO)=C2C)C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=N1 | 2959.6 | Standard non polar | 33892256 | | Thiamine,2TBDMS,isomer #2 | CC1=NC=C(C[N+]2=CSC(CCO)=C2C)C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=N1 | 3242.0 | Standard polar | 33892256 | | Thiamine,3TBDMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C(C)(C)C)=C2C)C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=N1 | 3094.1 | Semi standard non polar | 33892256 | | Thiamine,3TBDMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C(C)(C)C)=C2C)C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=N1 | 3195.6 | Standard non polar | 33892256 | | Thiamine,3TBDMS,isomer #1 | CC1=NC=C(C[N+]2=CSC(CCO[Si](C)(C)C(C)(C)C)=C2C)C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=N1 | 3187.4 | Standard polar | 33892256 |
|
|---|
| Spectra |
|---|
| |
|---|
| Biological Properties |
|---|
| Cellular Locations | - Extracellular
- Membrane (predicted from logP)
- Mitochondria
|
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
- Urine
|
|---|
| Tissue Locations | - Adipose Tissue
- Adrenal Gland
- Brain
- Erythrocyte
- Fibroblasts
- Intestine
- Kidney
- Liver
- Neuron
- Placenta
- Skeletal Muscle
- Testis
|
|---|
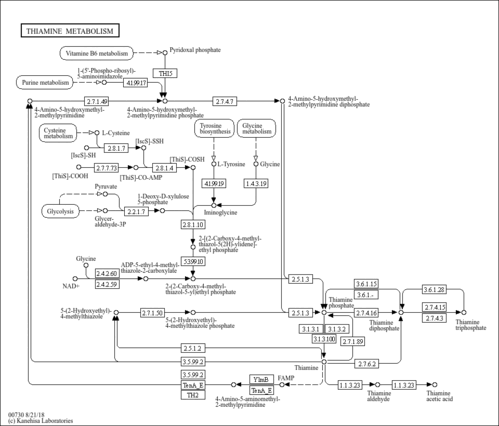
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.096 +/- 0.018 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.050-0.120 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.34 (0.14-0.79) uM | Newborn (0-30 days old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.16 (0.06-0.41) uM | Children (1-13 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.12 (0.094-0.280) uM | Adult (>18 years old) | Female | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.141 +/- 0.0543 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0487 +/- 0.00275 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.006 (0.005 - 0.007) uM | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 0.247 +/- 0.006 uM | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.12 +/- 0.09 umol/mmol creatinine | Children (1-13 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.02 +/- 0.01 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.22 (0.20-0.60) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.132 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.0283 +/- 0.0191 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.0511 +/- 0.0255 umol/mmol creatinine | Children (1 - 13 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.035 +/- 0.006 uM | Elderly (>65 years old) | Both | Hemodialysis | | details | | Blood | Detected and Quantified | 0.18 +/- 0.066 uM | Adult (>18 years old) | Both | Friedreich's ataxia | | details | | Blood | Detected and Quantified | 0.17 +/- 0.051 uM | Adult (>18 years old) | Both | Olivopontocerebellar atrophy (OPCA) | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.115 +/- 0.0464 uM | Adult (>18 years old) | Both | Friedreich's ataxia | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0902 +/- 0.0411 uM | Adult (>18 years old) | Both | Olivopontocerebellar atrophy (OPCA) | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.02 +/- 0.00 uM | Adult (>18 years old) | Not Specified | Alcoholism | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.01 +/- 0.00 uM | Adult (>18 years old) | Not Specified | Alcoholism | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.09 +/- 0.01 uM | Adult (>18 years old) | Not Specified | Olivopontocerebellar atrophy (OPCA) | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.06 +/- 0.01 uM | Adult (>18 years old) | Not Specified | Autosomal recessive spastic ataxia of Charlevoix-Saguena | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.1 +/- 0.01 uM | Not Specified | Not Specified | Friedreich's ataxia | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Hemodialysis |
|---|
- Hung SC, Hung SH, Tarng DC, Yang WC, Chen TW, Huang TP: Thiamine deficiency and unexplained encephalopathy in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2001 Nov;38(5):941-7. [PubMed:11684545 ]
| | Friedreich's ataxia |
|---|
- Pedraza OL, Botez MI: Thiamine status in inherited degenerative ataxias. J Neurol Neurosurg Psychiatry. 1992 Feb;55(2):136-7. [PubMed:1538220 ]
- Botez MI, Young SN: Biogenic amine metabolites and thiamine in cerebrospinal fluid in heredo-degenerative ataxias. Can J Neurol Sci. 2001 May;28(2):134-40. [PubMed:11383938 ]
| | Olivopontocerebral atrophy |
|---|
- Pedraza OL, Botez MI: Thiamine status in inherited degenerative ataxias. J Neurol Neurosurg Psychiatry. 1992 Feb;55(2):136-7. [PubMed:1538220 ]
- Botez MI, Young SN: Biogenic amine metabolites and thiamine in cerebrospinal fluid in heredo-degenerative ataxias. Can J Neurol Sci. 2001 May;28(2):134-40. [PubMed:11383938 ]
| | Alcoholism |
|---|
- Dastur DK, Santhadevi N, Quadros EV, Avari FC, Wadia NH, Desai MN, Bharucha EP: The B-vitamins in malnutrition with alcoholism. A model of intervitamin relationships. Br J Nutr. 1976 Sep;36(2):143-59. [PubMed:182198 ]
| | Hereditary spastic paraplegia |
|---|
- Botez MI, Young SN: Biogenic amine metabolites and thiamine in cerebrospinal fluid in heredo-degenerative ataxias. Can J Neurol Sci. 2001 May;28(2):134-40. [PubMed:11383938 ]
| | Colorectal cancer |
|---|
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
|
|
|---|
| Associated OMIM IDs | - 229300 (Friedreich's ataxia)
- 182601 (Hereditary spastic paraplegia)
- 114500 (Colorectal cancer)
|
|---|
| External Links |
|---|
| DrugBank ID | DB00152 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB008424 |
|---|
| KNApSAcK ID | C00000775 |
|---|
| Chemspider ID | 1098 |
|---|
| KEGG Compound ID | C00378 |
|---|
| BioCyc ID | THIAMINE |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Thiamine |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 1130 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 18385 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | THM |
|---|
| MarkerDB ID | MDB00000114 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Sugimoto, Hirohiko; Ishiba, Teruyuki; Sato, Tomohiro; Nakai, Hiroshi; Hirai, Kentaro. Novel S-alkylation products from "isolated thiamin ylide" via thiaminium neothiaminthiolate ion pair. Journal of Organic Chemistry (1990), 55(2), 467-70. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Dutta B, Huang W, Molero M, Kekuda R, Leibach FH, Devoe LD, Ganapathy V, Prasad PD: Cloning of the human thiamine transporter, a member of the folate transporter family. J Biol Chem. 1999 Nov 5;274(45):31925-9. [PubMed:10542220 ]
- Bellazzi R, Guglielmann R, Ironi L, Patrini C: A hybrid input-output approach to model metabolic systems: an application to intracellular thiamine kinetics. J Biomed Inform. 2001 Aug;34(4):221-48. [PubMed:11977806 ]
- Pietrzak I, Baczyk K: Comparison of the thiamine level in blood and erythrocyte transketolase activity in hemodialyzed and nondialyzed patients during recombinant human erythropoietin therapy. Miner Electrolyte Metab. 1997;23(3-6):277-82. [PubMed:9387133 ]
- Singleton CK, Martin PR: Molecular mechanisms of thiamine utilization. Curr Mol Med. 2001 May;1(2):197-207. [PubMed:11899071 ]
- Sato Y, Nakagawa M, Higuchi I, Osame M, Naito E, Oizumi K: Mitochondrial myopathy and familial thiamine deficiency. Muscle Nerve. 2000 Jul;23(7):1069-75. [PubMed:10883001 ]
- Mastrogiacoma F, Bettendorff L, Grisar T, Kish SJ: Brain thiamine, its phosphate esters, and its metabolizing enzymes in Alzheimer's disease. Ann Neurol. 1996 May;39(5):585-91. [PubMed:8619543 ]
- Molina JA, Jimenez-Jimenez FJ, Hernanz A, Fernandez-Vivancos E, Medina S, de Bustos F, Gomez-Escalonilla C, Sayed Y: Cerebrospinal fluid levels of thiamine in patients with Alzheimer's disease. J Neural Transm (Vienna). 2002 Jul;109(7-8):1035-44. [PubMed:12111441 ]
- Pietrzak I, Baczyk K, Kubiak W: Recombinant human erythropoietin administration improves thiamine content in blood and erythrocytes transketolase activity in pre-dialyzed patients. Ann Univ Mariae Curie Sklodowska Med. 1994;48 Suppl 3:29-37. [PubMed:8192530 ]
- Valerio G, Franzese A, Poggi V, Patrini C, Laforenza U, Tenore A: Lipophilic thiamine treatment in long-standing insulin-dependent diabetes mellitus. Acta Diabetol. 1999 Jun;36(1-2):73-6. [PubMed:10436256 ]
- Herve C, Beyne P, Delacoux E: Determination of thiamine and its phosphate esters in human erythrocytes by high-performance liquid chromatography with isocratic elution. J Chromatogr B Biomed Appl. 1994 Mar 4;653(2):217-20. [PubMed:8205249 ]
- Losa R, Sierra MI, Fernandez A, Blanco D, Buesa JM: Determination of thiamine and its phosphorylated forms in human plasma, erythrocytes and urine by HPLC and fluorescence detection: a preliminary study on cancer patients. J Pharm Biomed Anal. 2005 Apr 29;37(5):1025-9. [PubMed:15862682 ]
- Pietrzak I, Baczyk K, Mlynarczyk M: [The influence of intermittent peritoneal dialysis on free and total thiamine concentration in plasma and erythrocytes of patients with end stage renal disease]. Pol Arch Med Wewn. 1994;92 Spec No:31-6. [PubMed:7731897 ]
- Pedraza OL, Botez MI: Thiamine status in inherited degenerative ataxias. J Neurol Neurosurg Psychiatry. 1992 Feb;55(2):136-7. [PubMed:1538220 ]
- Vinogradov VV, Tarasov IuA, Tishin VS, Bogdanovich VI, Spas VV: [Thiamine prevention of the corticosteroid reaction afer surgery]. Probl Endokrinol (Mosk). 1981 May-Jun;27(3):11-6. [PubMed:7291135 ]
- Tanaka T, Sohmiya K, Kono T, Terasaki F, Horie R, Ohkaru Y, Muramatsu M, Takai S, Miyazaki M, Kitaura Y: Thiamine attenuates the hypertension and metabolic abnormalities in CD36-defective SHR: uncoupling of glucose oxidation from cellular entry accompanied with enhanced protein O-GlcNAcylation in CD36 deficiency. Mol Cell Biochem. 2007 May;299(1-2):23-35. [PubMed:16645728 ]
- Bettendorff L, Mastrogiacomo F, Kish SJ, Grisar T: Thiamine, thiamine phosphates, and their metabolizing enzymes in human brain. J Neurochem. 1996 Jan;66(1):250-8. [PubMed:8522961 ]
- Lee DC, Chu J, Satz W, Silbergleit R: Low plasma thiamine levels in elder patients admitted through the emergency department. Acad Emerg Med. 2000 Oct;7(10):1156-9. [PubMed:11015250 ]
- Shimon I, Almog S, Vered Z, Seligmann H, Shefi M, Peleg E, Rosenthal T, Motro M, Halkin H, Ezra D: Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am J Med. 1995 May;98(5):485-90. [PubMed:7733128 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|